- Drug Discovery
- Devise plans and strategies for optimized screening paradigms
- Pharmacokinetic (PK) / Toxicokinetic (TK)
- Protocol generation for in vitro and in vivo pharmacokinetic studies
- Data analysis and interpretation
- Margin of Efficacy (MoE)
- Toxicity enabling studies
- Support for PD studies
- Recommendations of dose selection for PD studies
- Frequency of dosing
- Integration between PK and efficacy
- In vivo DDI
- Provide advice on the interpretation of preclinical DDI studies
- Modeling and Simulation
- Non-compartmental and compartmental PK/PD modeling and analysis
- Predictive assessment
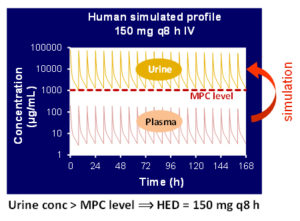
- Prediction of urine and tissue PK profile of test compound
- Human clinical dose estimation
- Regulatory submissions
- Pharmacokinetic & Toxicokinetic sections of IND and investigator brochures
- Reviews
- Data, documents, protocols, report and scientific literature
Preclinical model based drug development
A model-based approach to predict the pharmacokinetic profile of a drug in humans prior to the first human exposure by utilizing available in vitro and/or in vivo data. These early predictions about clinical efficacious dose and plasma / urine profile in human, illuminate drug development strategies and improve decision-making.